Aging is a complex, multi-layered process, and not everyone ages at the same pace. For decades, scientists have sought to identify objective ways to track biological aging, beyond counting the number of candles on a birthday cake. Biomarkers of aging—or “aging biomarkers”—offer invaluable insight into how quickly our cells, tissues, and physiological systems are declining. But what exactly are biomarkers of aging, how reliable are they, and how might they guide personalized strategies for healthy longevity?
What Are Biomarkers of Aging?
Biomarkers of aging are measurable biological indicators that reflect an individual’s physiological age—or the rate of biological decline—regardless of their chronological age. While chronological age is simply how many years you’ve lived, biological age aims to capture your body’s functional state and risk for age-related diseases.
Key characteristics of aging biomarkers:
- Objective and measurable: Can be quantified reliably in the lab or clinic.
- Reflect aging processes: Directly linked to mechanisms of biological aging (not just age-correlated).
- Predictive: Help forecast risk for age-related diseases, disabilities, or mortality.
Why Do We Need Biomarkers for Aging?
Measuring biological age helps clinicians and researchers:
- Track how quickly an individual is aging
- Evaluate the effectiveness of anti-aging therapies
- Identify at-risk populations for clinical intervention
- Personalize healthcare and lifestyle recommendations
- Advance understanding of the fundamental mechanisms of aging
Major Categories of Aging Biomarkers
Aging touches every part of the body, so scientists have developed a wide range of biomarkers. Below are the most validated and promising tools for measuring biological age:
1. Epigenetic Clocks
Epigenetic clocks are calculated from DNA methylation patterns—chemical modifications on DNA that change predictably with age. The best-known example, the Horvath Clock, analyses methylation at hundreds of sites across the genome to estimate biological age.
- Advantages: Highly accurate, applicable to multiple tissues
- Limitations: Influenced by disease status, environment, and sample type
2. Telomere Length
Telomeres are protective DNA caps at the ends of chromosomes that shorten with every cell division. Critically short telomeres can signal cell senescence—a hallmark of aging.
- Advantages: Simple blood test, predictive of mortality risk
- Limitations: High variability between individuals, not always aligned with health outcomes
3. Blood-Based Biomarker Panels
Panels combining easily measured blood markers provide practical tools for clinics. These can include:
- C-reactive protein (CRP): Chronic inflammation marker
- Glycated hemoglobin (HbA1c): Glucose control over time
- Creatinine, albumin, white blood cell count: Reflect organ function and general health
Composite indices like the PhenoAge and GrimAge scores aggregate multiple blood biomarkers for a more robust estimate of biological aging.
4. Proteomic and Metabolomic Signatures
With advances in high-throughput technology, it is now possible to assess hundreds of proteins or metabolites in the blood to generate aging profiles.
- Proteomics: Aging is associated with changes in inflammation-related and tissue remodeling proteins.
- Metabolomics: Metabolite patterns reflecting mitochondrial function, fat metabolism, and oxidative stress change as we age.
5. Functional Biomarkers
Practical tests of organ and system function often correlate well with aging and frailty:
- Gait speed
- Grip strength
- Cognitive screening tests (e.g., MMSE)
- Cardiorespiratory fitness tests
Combining functional and molecular measures yields a comprehensive view of aging.

Mechanisms Underlying Aging Biomarkers
Understanding how biomarkers track aging begins with the biology itself. Aging involves several interconnected processes, including:
- Genomic instability (DNA damage accumulation)
- Epigenetic alterations (shifts in DNA methylation and histone markers)
- Loss of proteostasis (protein aggregation or breakdown)
- Cellular senescence (cells losing function and releasing inflammatory signals)
- Mitochondrial dysfunction (energy production drop)
- Altered intercellular communication (chronic inflammation or inflammaging)
Biomarkers like epigenetic clocks and proteomic signatures are designed to capture these hallmarks, helping bridge molecular changes with biological decline.
Validating Biomarkers: Challenges and Developments
The gold standard for aging biomarkers:
- Predict mortality, disease risk, or disability better than chronological age alone
- Change in response to interventions (lifestyle, pharmacological, etc.)
- Replicable across populations, ethnicities, and environments
However, real-world limitations persist:
- Heterogeneity: Aging varies greatly between individuals.
- Environmental Effects: Lifestyle, pollution, and diet can shift biomarker levels, separating cause from correlation.
- Tissue specificity: Biomarkers may behave differently in different tissues (e.g., blood, skin, liver).
- Technical limitations: Some methods are expensive, complex, or lack clinical validation.
No single biomarker is perfect; using panels and composite scores improves accuracy.
Aging Biomarker Applications in Research and Medicine
Clinical Trials
Pharmaceutical and nutrition studies are increasingly using aging biomarkers as endpoints in trials of anti-aging compounds and interventions. These allow:
- Detection of subtle biological effects in short periods
- Earlier insight into risk or benefits, before overt disease develops
Learn about ongoing research: Clinical Trials in Age-Related Degenerative Conditions
Risk Stratification and Prevention
Biomarker panels can identify individuals at increased risk for age-related chronic diseases (cardiovascular disease, diabetes, dementia) and help tailor preventive strategies.
Personalized Medicine
Tracking biological age enables clinicians to:
- Assess whether a patient is aging more rapidly than peers
- Guide screening and intervention choices
- Motivate behavioral change with objective feedback
Monitoring Lifestyle and Anti-Aging Interventions
Researchers are exploring whether positive lifestyle changes—such as diet, exercise, and stress reduction—or novel compounds can actually slow or reverse biological aging, as measured by validated biomarkers.

Practical Caveats and Safety Considerations
- Interpret with Caution: A single biomarker result rarely tells the entire story. Medical decisions should rely on a comprehensive clinical assessment.
- Variability: Genetics, race, and even the laboratory running the test may influence results.
- Not Diagnostic: Most biomarkers are not used to diagnose specific diseases, but rather indicate general risk or biological status.
- Unknown Implications: Lowering biological age scores may not always translate to improved health or longer life spans. More long-term research is needed.
Current and Emerging Aging Biomarkers: Summary Table
| Approach | Example Tests | Human Evidence | Strengths | Limitations |
|---|---|---|---|---|
| Epigenetic Clocks | Horvath, Hannum, GrimAge | Strong | Highly precise, predictive | Expensive, still evolving |
| Telomere Length | Leukocyte telomere measurement | Moderate | Widely available | Variable, less specific |
| Blood Biomarker Panels | PhenoAge, CRP, albumin, WBC, etc. | Strong | Practical, low cost | May miss non-blood aspects |
| Proteomic/Metabolomic Tests | Protein/metabolite age signatures | Emerging | High-resolution, pathway insight | Specialized, costly |
| Functional Biomarkers | Gait, grip, cognition, VO2 | Strong | Easy to collect, established | Non-specific, environmental |
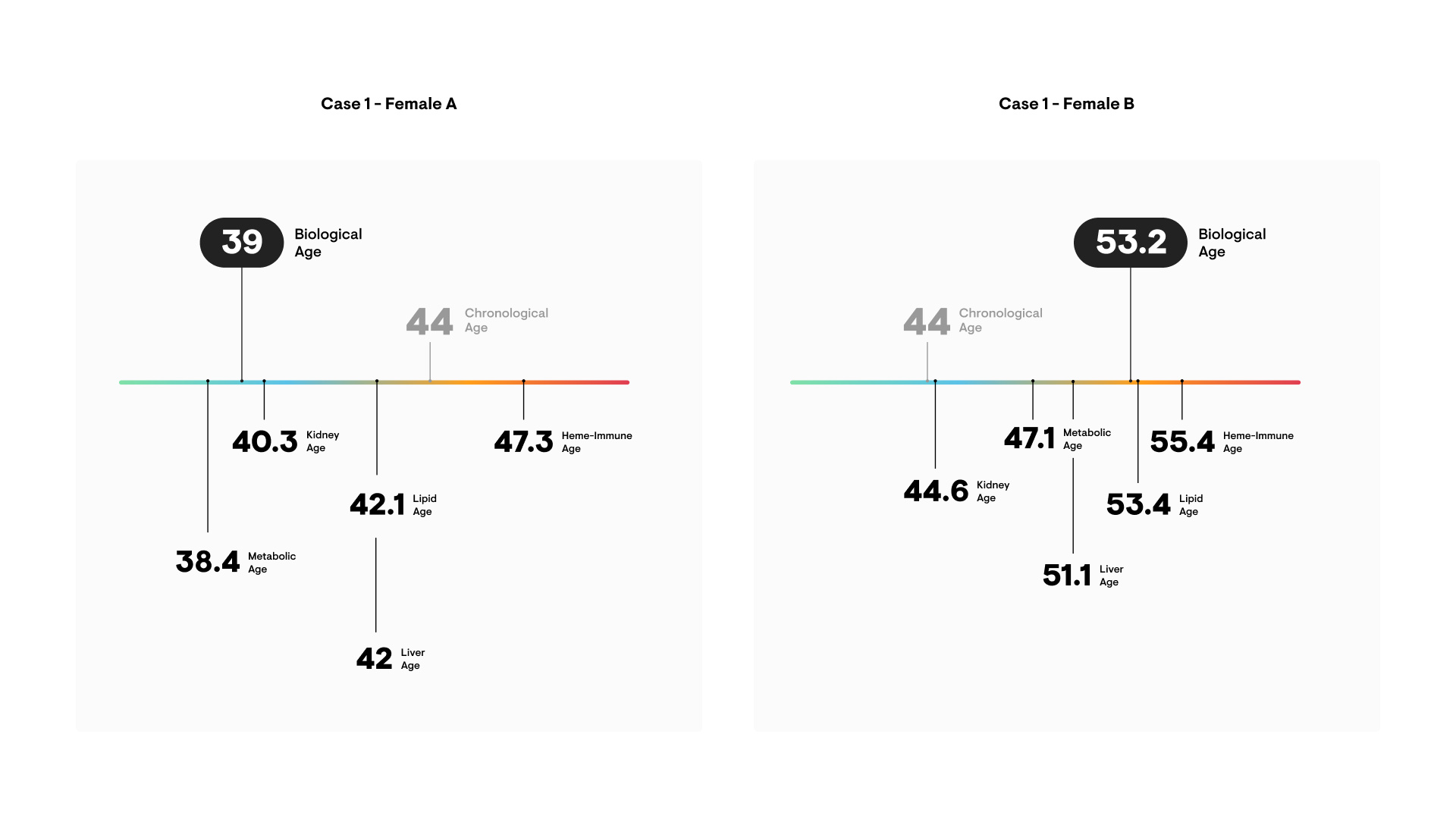
Individual Variability: Why Not All Aging Biomarkers Agree
Some people show younger biological ages than peers of the same chronological age, and vice versa. Factors influencing biomarker results include:
- Genetic variation
- Chronic illnesses
- Lifestyle choices (smoking, exercise, nutrition)
- Socioeconomic and environmental conditions
This underpins the movement toward “precision aging”—the idea that one-size-fits-all measures are less effective than personalized, multifaceted approaches.
The Future of Aging Biomarker Science
As technology advances, the field is moving toward:
- Multi-omics integration: Linking genomics, epigenomics, proteomics, and metabolomics for deeper insights
- Machine learning: Developing algorithms to refine risk prediction and optimal intervention timing
- Widespread clinical use: Making aging biomarker panels available in routine healthcare
Key Takeaways:
- Biomarkers aging research is transforming how we measure, understand, and intervene in biological aging.
- Reliable, validated aging biomarkers help predict health risks, track interventions, and may one day guide anti-aging therapies.
- The most accurate approach is to combine molecular, functional, and clinical measures.
Studies / References
- Horvath S.
DNA methylation age of human tissues and cell types.
Genome Biology. 2013;14(10):R115.
https://pubmed.ncbi.nlm.nih.gov/24138928/
Foundational paper introducing epigenetic clocks as accurate measures of biological age across tissues. - Levine ME, et al.
An epigenetic biomarker of aging for lifespan and healthspan (PhenoAge).
Aging (Albany NY). 2018;10(4):573–591.
https://pubmed.ncbi.nlm.nih.gov/29676998/
Demonstrates that composite epigenetic aging measures predict mortality and age-related disease better than chronological age. - Müezzinler A, Zaineddin AK, Brenner H.
A systematic review of leukocyte telomere length and age-related diseases.
Ageing Research Reviews. 2013;12(2):509–519.
https://pubmed.ncbi.nlm.nih.gov/23333817/
Comprehensive review showing telomere length as a biologically relevant but variable aging marker. - Tanaka T, et al.
Plasma proteomic signature of age and physical function.
Nature Medicine. 2018;24(12):1901–1911.
https://pubmed.ncbi.nlm.nih.gov/30478444/
Identifies protein signatures associated with functional decline and biological aging. - Studenski S, et al.
Gait speed and survival in older adults.
JAMA. 2011;305(1):50–58.
https://pubmed.ncbi.nlm.nih.gov/21205966/
Establishes gait speed as a powerful, practical functional biomarker of aging and mortality.Conclusion: Harnessing the Power of Aging Biomarkers
Understanding and deploying biomarkers aging science is key to revolutionizing prevention, healthcare, and personal longevity. As research develops, these tools will become ever more precise—helping us measure, track, and perhaps one day slow the biological age of every individual.