Ligandrol (LGD-4033) is increasingly discussed in medical research circles as a selective androgen receptor modulator (SARM) with potential applications in muscle preservation, especially against the backdrop of age-related muscle loss. As the global population ages, sarcopenia and muscle degeneration become pressing health concerns. There is growing interest in new therapies and compounds that safely mitigate muscle loss in older adults. In this article, we comprehensively examine Ligandrol’s human evidence, mechanism of action, safety, potential for SARM therapy, and the intricate landscape of muscle health and aging.
What is Ligandrol (LGD-4033)?
Ligandrol, also known as LGD-4033, is a non-steroidal selective androgen receptor modulator (SARM). SARMs are a class of compounds that bind to androgen receptors with greater tissue selectivity than traditional anabolic steroids. Ligandrol was developed to fight muscle wasting in serious medical conditions, such as:
- Age-related sarcopenia (progressive loss of muscle mass)
- Cachexia (muscle loss due to cancer or chronic illness)
- Physical immobility, including after surgery or trauma
Unlike testosterone, SARMs like LGD-4033 aim to stimulate muscle growth without causing significant androgenic or estrogenic side effects in other tissues.
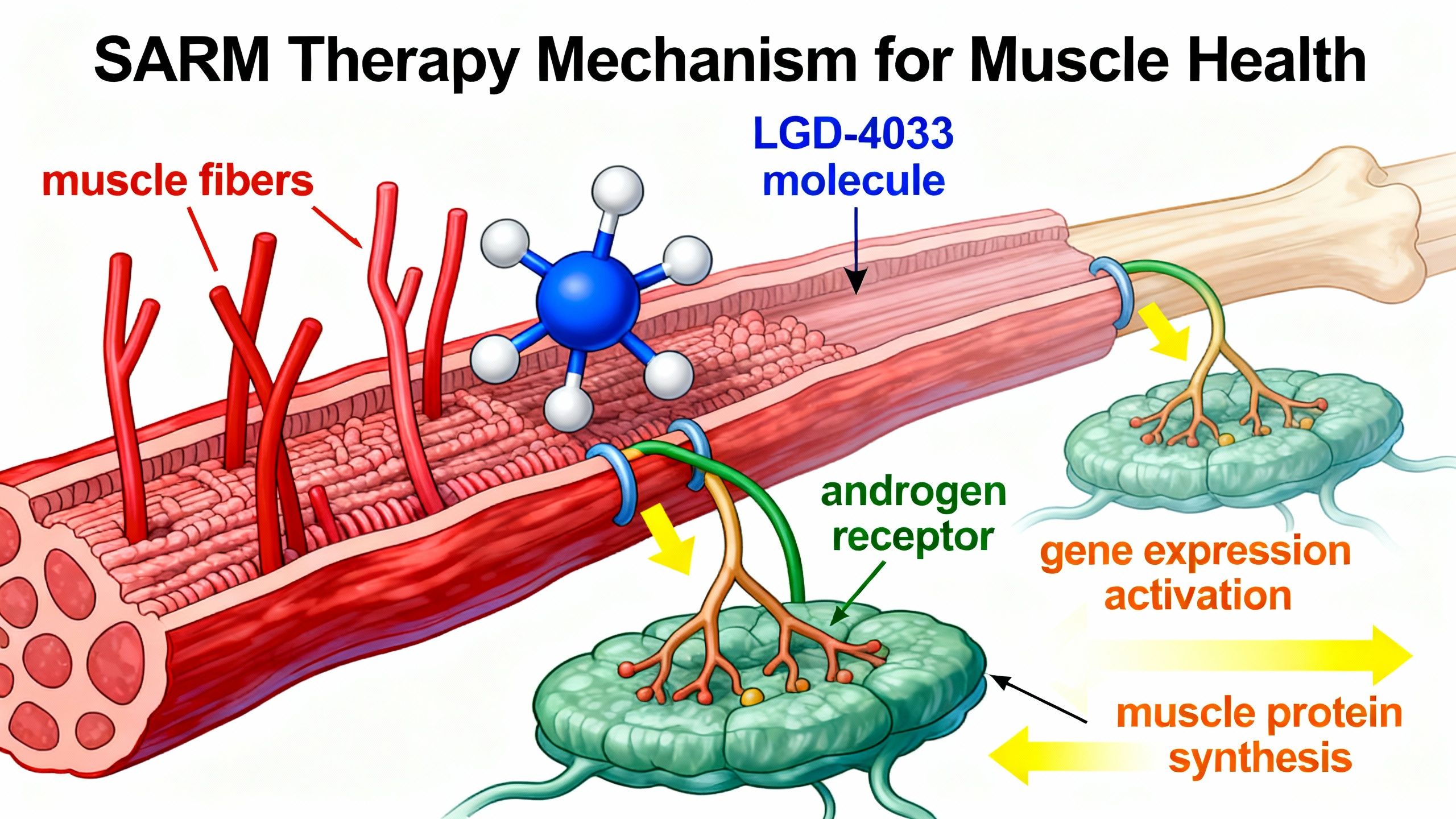
Mechanism of Action: How Does Ligandrol Work?
SARMs, including Ligandrol, exert their effects by selectively binding to androgen receptors in skeletal muscle and bone. This specificity distinguishes them from traditional anabolic steroids, which also act on the prostate, skin, and hair follicles.
Key Mechanisms:
- Tissue Selectivity: LGD-4033 preferentially binds receptors in muscle and bone, achieving anabolic effects with fewer off-target actions.
- Muscle Protein Synthesis: Ligandrol increases protein synthesis, which is fundamental for muscle repair, hypertrophy, and preservation.
- Bone Density Support: By activating androgen receptors in bone, LGD-4033 may enhance bone mineral density, potentially lowering osteoporosis risk when combined with established therapies.
Key finding: Human trials indicate that LGD-4033 can significantly increase lean muscle mass over a short timeframe without major hormonal disturbances in healthy men.
Human Research on Ligandrol: What Does the Evidence Show?
Summary Table: Key Human Studies on Ligandrol
| Study Population | Duration | Key Outcomes | Main Limitations |
|---|---|---|---|
| Healthy young men (Phase I) | 3 weeks | Increased lean muscle mass; well tolerated | Short duration, no elderly subjects |
| Older adults (Phase II; ongoing) | 12+ weeks | Data pending; primary endpoint: muscle strength/function | Data unpublished as of 2024 |
| Cancer cachexia patients | Not yet published | Studied for muscle wasting prevention | Limited published data |
Detailed Study Summaries
- Study in Healthy Young Men
- Ongoing Research in Older Adults
- Investigational Use in Cancer Cachexia
Ligandrol in the Context of Aging: Addressing Sarcopenia
Sarcopenia refers to the gradual loss of muscle mass and strength associated with advancing age. It is a significant contributor to frailty, falls, loss of independence, and increased mortality in the elderly. Traditional management strategies focus on nutrition, resistance exercise, and certain pharmacological interventions.
How Might Ligandrol Help?
- Anabolic support: By increasing muscle protein synthesis and reducing breakdown, Ligandrol may help preserve muscle mass in populations at risk of sarcopenia.
- Improved function: Increased muscle mass, in theory, translates to improved strength and physical capability—although long-term evidence in seniors is still limited.
- Adjunct to mobility therapy: Used alongside physiotherapy, SARMs may enable greater gains from exercise in individuals unable to participate in strenuous activity.

Important Caveats
- Evidence is emerging: While healthy young men experienced benefits, clinical trials in older adults and those with chronic conditions remain in early stages.
- Long-term safety is unknown: No studies have followed participants beyond a few months, so risks of prolonged use, especially in aging populations, are unclear.
- Best used in a supervised medical context: All investigational data recommend specialist supervision.
Practical Considerations & Dosage in Human Studies
Research to date has explored LGD-4033 in doses ranging from 0.1 mg to 1 mg per day, usually for 3–6 weeks.
Key points from human dosing:
- Low doses produced measurable muscle gains without marked adverse effects.
- No significant effect on prostate-specific antigen (PSA) or estradiol at studied doses.
- Endogenous testosterone decreased only slightly or was unaffected at low doses, emphasizing tissue selectivity.
Note: Clinical efficacy and safety at longer durations and in frail populations are unknown. Self-experimentation outside of clinical trials is strongly discouraged.
Ligandrol vs. Ostarine and Other SARMs: Comparative Evidence
Ligandrol and Ostarine (MK-2866) are among the most researched SARMs for muscle preservation. Early evidence suggests:
- Ligandrol may produce slightly greater increases in lean mass over 3–4 weeks compared to Ostarine in healthy volunteers.
- Side effect profiles are similar at doses ≤1 mg.
- No SARM is FDA-approved for muscle wasting or anti-aging.
Safety Profile & Potential Side Effects
What have human studies found?
- Generally well-tolerated in the short-term (up to 3 weeks)
- Mild, reversible changes in blood lipid profiles
- Slight, transient suppression of natural testosterone at higher doses
- No prostate, liver, or cardiovascular toxicity seen at standard trial doses
Major Caveats
- Long-term safety data are lacking
- Potential for unknown side effects with prolonged or high-dose use
- Liver function should be monitored in clinical contexts
While SARMs are sometimes available as non-prescription products online, purity and dosing are unregulated, posing substantial health risks.

Drug Interactions, Contraindications, and Use in Special Populations
Drug Interactions
- No major clinically significant drug-drug interactions have been documented in published studies.
- Theoretical interactions may exist with:
- CYP3A4 inhibitors/inducers
- Medications affecting androgen pathways
Contraindications
- History of hormone-sensitive cancers (e.g., prostate, breast, endometrial)
- Pregnant or breastfeeding women
- Patients with severe liver disease
Clinical Trial Populations
- Most studies have excluded individuals with pre-existing cardiovascular, hepatic, or renal disease. Thus, safety in these groups is unknown.
The Research Context: SARM Therapy in Medicine
Pharmaceutical-grade SARMs are not intended for over-the-counter human use and are not FDA-approved for muscle wasting or anti-aging indications.
- Off-label or unsupervised use has been associated with dangerous adverse reactions and product contamination.
- SARMs are banned in professional sports for their anabolic potential.
- Medical use should occur only in trial or specialist settings, strictly following regulatory guidance.
Questions and Future Directions: What Remains Unanswered?
Despite clear anabolic effects in short-term human studies, several critical unknowns must be addressed:
- Long-term safety: No data beyond several months.
- Sustainability of gains: Do muscle increases persist post-use?
- Relevance in diverse populations: Minimal data in women, elderly, or patients with comorbidities.
- Risk of misuse: Growing off-label and non-medical use raises public health concerns.
- Potential as a clinical tool: Ongoing trials will determine if SARM therapy can be a safe, regulated approach for select patients.
Conclusion: Where Does Ligandrol Fit in Muscle Health and Sarcopenia?
Ligandrol (LGD-4033) demonstrates clear short-term efficacy in increasing lean muscle mass and appears generally well-tolerated in healthy men. Its potential applications in aging-related muscle loss and SARM therapy remain promising yet still unproven in vulnerable populations, such as older adults and those with chronic disease.
Until large, long-term, peer-reviewed studies are available, Ligandrol should be considered an investigational therapy, best restricted to clinical trials or specialist use. Future research will clarify the full potential and risks of this compound in fighting sarcopenia and enhancing muscle health during aging.
Studies / References
- Basaria, S., et al. (2013). The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral selective androgen receptor modulator, in healthy young men. The Journals of Gerontology: Series A. Retrieved from PubMed.
- LGD-4033 Phase I Safety & Lean Mass Data. (2013). PMC4111291.
- LGD-4033 Clinical Studies (Phase II Hip Fracture). Wikipedia.
- Chen, J., Kim, J., & Dalton, J.T. (2005). Discovery and therapeutic promise of selective androgen receptor modulators. Molecular Interventions, 5(3). DOI:10.1124/mi.5.3.7.
- LGD-4033 Drug-Induced Liver Injury Case Report. PubMed.
Note: Only human studies included. Animal/in-vitro data was referenced for background only, not core conclusions.
Important: This article is for informational purposes only. If considering any investigational therapy for muscle loss or age-related conditions, consult a healthcare provider or participate in legitimate clinical trials. Self-medication with unregulated compounds like SARMs can be dangerous.



