In the relentless pursuit of interventions that can mitigate the negative aspects of aging, the scientific community has identified cellular senescence as a key target. Senescent cells, often described as “zombie cells,” accumulate in tissues as we age, contributing to a wide range of age-related diseases and functional decline. The quest for compounds that can selectively eliminate these cells—a strategy known as senolytic therapy—has led to the development of novel molecules, one of the most discussed being FOXO4-DRI.
FOXO4-DRI is a synthetic peptide designed with a specific purpose: to disrupt a key survival pathway in senescent cells, triggering their self-destruction. While it has generated significant excitement due to its remarkable effects in animal models, it represents the cutting edge of experimental aging research. This article provides a comprehensive, evidence-based overview of FOXO4-DRI, exploring its mechanism of action, the preclinical evidence, its potential applications, and the critical safety considerations surrounding this unapproved research compound.
The Problem of Cellular Senescence in Aging
To understand the purpose of a senolytic peptide like FOXO4-DRI, one must first grasp the concept of cellular senescence. Far from being a dormant state, senescence is an active, complex biological process with profound implications for the aging body.
What Are Senescent Cells?
Cellular senescence is a fate that cells can enter in response to various forms of stress or damage, such as DNA damage, telomere shortening, or oncogenic signaling. When a cell becomes senescent, it permanently exits the cell cycle, meaning it can no longer divide and replicate. This is a crucial protective mechanism that prevents potentially cancerous cells from proliferating, acting as a powerful tumor suppressor.
Initially, this process is beneficial. However, as we age, the immune system’s ability to clear these senescent cells diminishes. This leads to their progressive accumulation in virtually all tissues, including the skin, kidneys, lungs, heart, and brain. These lingering cells are not merely inactive bystanders; they actively secrete a cocktail of inflammatory molecules that degrade the surrounding tissue environment.
The Senescence-Associated Secretory Phenotype (SASP)
The harmful effects of senescent cells are largely mediated by the Senescence-Associated Secretory Phenotype, or SASP. This refers to the complex mixture of pro-inflammatory cytokines, chemokines, growth factors, and proteases that senescent cells release into their local environment.
The SASP creates a state of chronic, low-grade inflammation, often termed “inflammaging.” This inflammatory milieu can:
- Induce senescence in neighboring healthy cells, creating a domino effect that accelerates the aging process.
- Degrade the extracellular matrix, the structural scaffolding that gives tissues their integrity and form.
- Attract immune cells, further fueling inflammation.
- Impair tissue regeneration and stem cell function.
This chronic secretion is a primary driver of many age-related conditions, from osteoarthritis and atherosclerosis to neurodegeneration and metabolic dysfunction. Therefore, the central hypothesis of senolytic therapy is that by selectively clearing senescent cells, we can reduce the SASP burden and, in turn, alleviate or even reverse aspects of age-related pathology.
Introducing FOXO4-DRI: A Novel Senolytic Peptide
FOXO4-DRI emerged from this scientific context as a highly targeted approach to eliminating senescent cells. Unlike some broader-acting senolytics, it was rationally designed to interfere with a specific protein interaction that is critical for the survival of senescent cells but not healthy, dividing cells.
What is a Peptide?
A peptide is a short chain of amino acids linked by peptide bonds. They are essentially small proteins. Peptides can act as signaling molecules, hormones, or, in the case of FOXO4-DRI, as therapeutic agents that modulate cellular processes. Because of their specificity and biological nature, peptides are an area of intense interest in medical research for developing targeted therapies.
The Discovery and Design of FOXO4-DRI
The development of FOXO4-DRI was based on a key observation about how senescent cells evade apoptosis, or programmed cell death. Healthy cells have mechanisms to self-destruct when they are damaged beyond repair. Senescent cells, despite being damaged, manage to resist this process.
Researchers discovered that a protein called FOXO4 (Forkhead box O4) was highly expressed and localized within the nucleus of senescent cells. There, it binds to another crucial protein, p53. The p53 protein is famously known as the “guardian of the genome” for its role in orchestrating DNA repair and apoptosis. In senescent cells, the binding of FOXO4 to p53 prevents p53 from initiating the apoptotic cascade. In essence, FOXO4 acts as a bodyguard for p53, keeping it sequestered and preventing it from triggering the cell’s self-destruct sequence.
FOXO4-DRI was engineered as a “decoy.” It is a modified peptide that includes a small, critical portion of the FOXO4 protein that is responsible for binding to p53. The “DRI” part of the name refers to a specific sequence (D-Retro-Inverso) that makes the peptide resistant to degradation by enzymes in the body, increasing its stability and bioavailability. When introduced into the body, FOXO4-DRI enters senescent cells and competitively binds to p53, displacing the native FOXO4 protein. This disruption frees p53 to initiate apoptosis, leading to the selective death of the senescent cell.
Mechanism of Action: How Does FOXO4-DRI Work?
The elegance of FOXO4-DRI lies in its highly specific mechanism, which exploits a vulnerability unique to senescent cells. This targeted approach is a significant goal in senolytic therapy, as it aims to minimize effects on healthy, non-senescent cells.
The p53 and FOXO4 Interaction
In a normal, healthy cell, the interaction between FOXO4 and p53 is not a primary survival mechanism. These cells rely on other pathways to regulate their life and death. However, in a senescent cell, this interaction becomes a critical dependency—an “Achilles’ heel.”
The cell has committed to senescence as a way to avoid becoming cancerous, but it must also avoid self-destructing. By tethering p53, the cell enters a state of suspended animation, unable to divide but also unable to die. This is the precise state that FOXO4-DRI is designed to interrupt.
Inducing Apoptosis in Senescent Cells
Apoptosis is an orderly, clean process of cellular suicide. When p53 is freed from its bond with FOXO4, it translocates to the mitochondria—the cell’s powerhouses—and activates a cascade of proteins, primarily from the BCL-2 family (like Bax and Bak), that initiate cell death.
This process involves the cell shrinking, its chromatin condensing, and its membrane blebbing. The cell then breaks apart into small, contained vesicles called apoptotic bodies, which are quickly cleaned up by immune cells called phagocytes. This is a non-inflammatory process, which is crucial. It means that clearing senescent cells via apoptosis does not, in itself, create further inflammation, unlike necrotic cell death, which is messy and inflammatory.
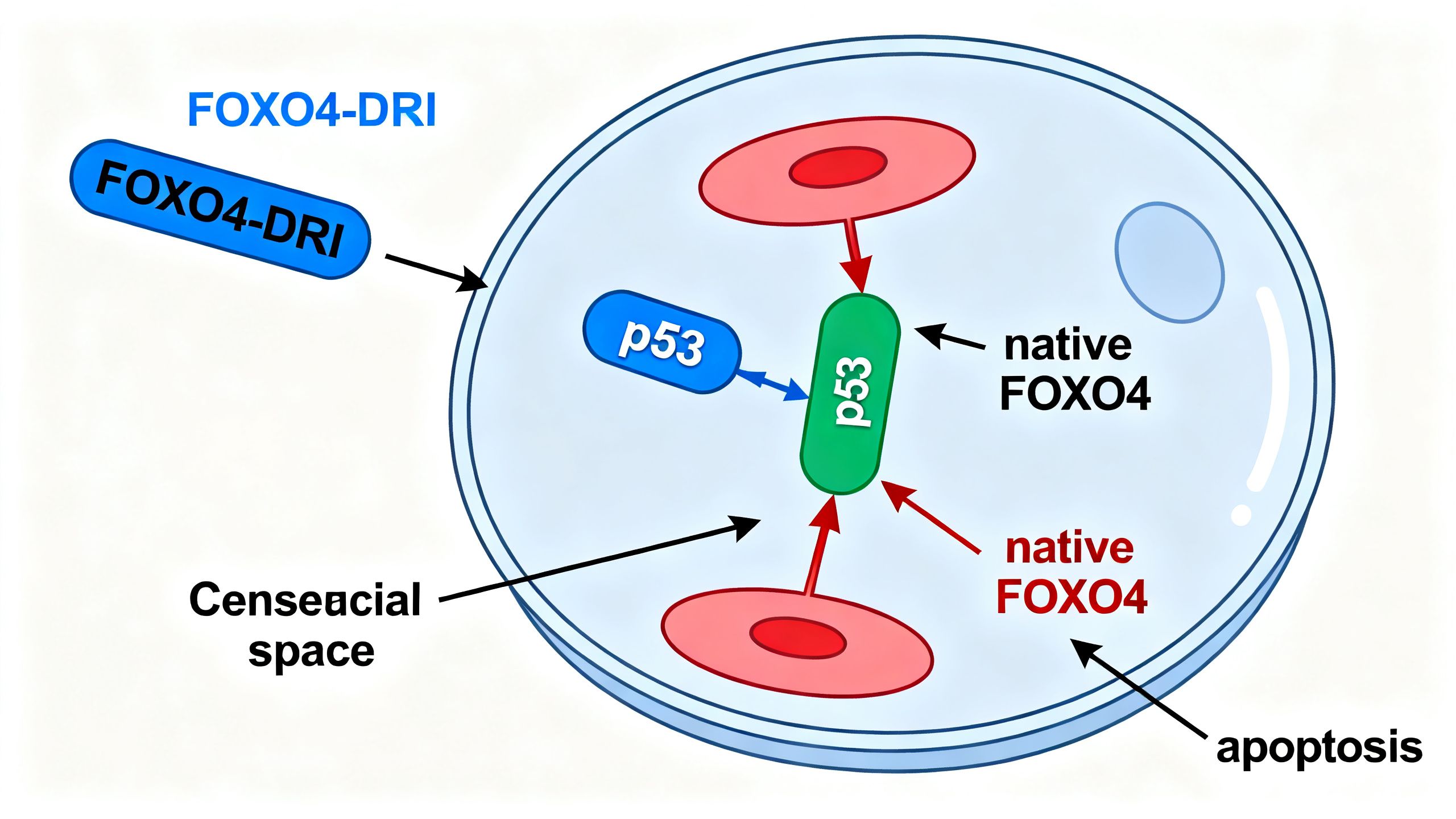
The Specificity of FOXO4-DRI
The theoretical specificity of this peptide for aging is a major point of interest. Because the FOXO4-p53 interaction is a key survival pathway primarily in senescent cells, FOXO4-DRI should, in principle, have little to no effect on healthy, proliferating cells or quiescent stem cells. This is a significant advantage over other senolytic agents that target more general survival pathways (like those in the BCL-2 family), which can sometimes lead to off-target effects on platelets or certain immune cells.
This specificity was demonstrated in preclinical studies where the peptide selectively killed senescent cells while leaving their non-senescent counterparts unharmed. However, it is crucial to remember that this specificity has only been confirmed in laboratory and animal models, not in rigorous human clinical trials.
Preclinical Evidence: What the Animal Studies Show
The excitement surrounding FOXO4-DRI is almost entirely based on a landmark 2017 study published in the journal Cell, along with subsequent preclinical research. These studies used mouse models of accelerated and natural aging to test the peptide’s effects. The results were striking and are widely cited as proof-of-concept for this senolytic peptide.
Reversal of Age-Related Decline in Mice
In naturally aged mice, systemic administration of FOXO4-DRI led to a significant clearance of senescent cells. This clearance was associated with remarkable functional improvements:
- Restored Fur Density: Aged mice often have a patchy, sparse coat. After treatment, they showed restored fur density, a visible sign of rejuvenation.
- Increased Physical Activity: Treated mice demonstrated increased exploratory behavior and voluntary wheel running, suggesting improved vitality and energy levels.
- Improved Kidney Function: Age-related decline in kidney function, measured by specific biomarkers, was reversed. The peptide restored renal health to levels seen in younger animals.
These effects were observed without apparent toxicity or adverse side effects in the mice. The treatment effectively turned back the clock on several physiological markers of aging.
Effects on Chemotherapy-Induced Senescence
Chemotherapy is a major inducer of cellular senescence. While it effectively kills cancer cells, it also damages healthy cells, pushing them into a senescent state. This is thought to be a primary cause of the debilitating, long-term side effects of chemotherapy, such as fatigue, organ damage, and accelerated aging.
In mouse models where senescence was induced by the chemotherapy drug doxorubicin, treatment with FOXO4-DRI was highly effective. It cleared the chemotherapy-induced senescent cells and mitigated the associated side effects. This suggests a potential therapeutic application for improving the quality of life and long-term health of cancer survivors.
The State of Human Research on FOXO4-DRI
This is the most critical section of the article for anyone considering this compound. Despite the extraordinary results in animal models, the translation from mouse to human is a long and often unsuccessful journey. As of late 2023, there are no published, peer-reviewed human clinical trials for FOXO4-DRI.
The Critical Gap: Lack of Published Human Trials
The absence of human data means that virtually everything about how FOXO4-DRI affects the human body is unknown. This includes:
- Efficacy: Does it effectively clear senescent cells in humans as it does in mice?
- Dosage: What is a safe and effective dose for humans?
- Safety and Side Effects: What are the short-term and long-term side effects? Does it have off-target effects on specific human cell types that weren’t apparent in mice?
- Pharmacokinetics: How is the peptide absorbed, distributed, metabolized, and excreted in the human body?
Without this information, any use of FOXO4-DRI in humans is pure experimentation with completely unknown risks. The history of medicine is filled with compounds that showed immense promise in animals but failed in human trials due to lack of efficacy or, worse, unforeseen toxicity.
Why Preclinical Results Don’t Always Translate
There are many reasons why mouse studies may not predict human outcomes. Laboratory mice are genetically homogenous and live in controlled environments, which is very different from the genetic diversity and varied lifestyles of the human population. Furthermore, the underlying biology of aging and disease can differ in subtle but crucial ways between species. A pathway that is a vulnerability in mouse senescent cells may not be as critical in human cells, or humans may have compensatory mechanisms that mice lack.
Safety, Side Effects, and Unknowns
Given the lack of human data, any discussion of safety is purely theoretical and must be approached with extreme caution. The use of unapproved research chemicals purchased from online vendors carries immense risks.
Theoretical Risks of Senolytic Therapy
Even if FOXO4-DRI works as intended, senolytic therapy itself has potential theoretical risks that need to be studied:
- Impaired Wound Healing: Senescent cells play a temporary, beneficial role in wound healing by secreting factors that help organize tissue repair. Eliminating them too aggressively could potentially impair this process.
- Embryogenesis: Certain developmental processes rely on senescent cells, so use during pregnancy would be extremely dangerous.
- Tumorigenesis: While senescence is a barrier to cancer, there is a theoretical concern that interfering with these pathways could, in some contexts, have unintended consequences related to cancer risk. The p53 protein, which FOXO4-DRI targets, is a master tumor suppressor, and modulating its function is a delicate matter.
The Dangers of Using Unapproved Research Chemicals
Peptides like FOXO4-DRI sold online are intended for research purposes only and are not manufactured to pharmaceutical-grade standards for human consumption. These products can have:
- Incorrect Purity: The product may contain less of the active peptide than advertised, or it could be contaminated with harmful impurities from the synthesis process.
- No Quality Control: There is no regulatory oversight to ensure the identity, strength, quality, and purity of these products.
- Risk of Infection: If the product is intended for injection, non-sterile manufacturing can lead to life-threatening bacterial infections.
A Critical Warning: Not for Human Consumption
Under no circumstances should individuals attempt to self-administer FOXO4-DRI or any other research chemical. The risks are unknown and potentially severe. Any discussion of this compound is for informational and educational purposes only, within the context of ongoing scientific research. It is not a recommendation or endorsement for use.

Comparing FOXO4-DRI to Other Senolytics
FOXO4-DRI is just one of several approaches being investigated for senescent cell clearance. It’s useful to compare it to other prominent senolytics to understand its unique place in this field of aging interventions.
- Dasatinib + Quercetin (D+Q): This is the most-studied senolytic combination in humans. Dasatinib is a chemotherapy drug, and Quercetin is a plant flavonoid. Together, they target different survival pathways in senescent cells. Unlike the highly specific FOXO4-DRI, D+Q is a blunter instrument and has known side effects.
- Fisetin: Another natural flavonoid, similar to Quercetin, found in fruits like strawberries. It has shown senolytic activity in animal studies and is now being tested in human clinical trials. It is thought to be safer than D+Q but its efficacy in humans is still under investigation.
- Navitoclax (ABT-263): A powerful senolytic that inhibits the BCL-2 family of anti-apoptotic proteins. It is very effective at clearing senescent cells but has significant toxicity, most notably causing a drop in platelet counts (thrombocytopenia), which has limited its use.
Compared to these, the main theoretical advantage of the FOXO4-DRI peptide for aging is its high specificity, which could translate to a better safety profile if it ever reaches human trials. Its main disadvantage is the complete lack of human data, whereas D+Q and Fisetin are already in clinical investigation.
Conclusion: The Promise and Peril of FOXO4-DRI
FOXO4-DRI represents a fascinating and potentially powerful tool in the future of aging science. As a rationally designed senolytic peptide, its ability to selectively induce apoptosis in senescent cells by disrupting the FOXO4-p53 interaction is a testament to our growing understanding of the molecular drivers of aging. The results from animal studies—showing restored organ function, increased vitality, and reversal of age-related phenotypes—are nothing short of remarkable and provide a compelling rationale for further research.
However, this promise is currently confined to the laboratory. The chasm between preclinical success and proven human therapy is vast and fraught with uncertainty. Without a single published human trial, the efficacy, safety, and dosage of FOXO4-DRI in humans remain entirely unknown. The allure of its reported effects in mice must be tempered by a strong dose of medical caution and scientific realism. The use of this compound outside of a rigorously controlled clinical trial is a dangerous gamble with unknown consequences. For now, FOXO4-DRI is a beacon of hope in the field of senolytic therapy, but its light shines from a distant shore that can only be reached through careful and methodical clinical investigation.
Key Preclinical Studies & References
Disclaimer: All studies listed below are preclinical (animal or in vitro). As of publication, there are no peer-reviewed human clinical trials evaluating FOXO4-DRI.
FOXO4-DRI–Specific Research
Baar, M. P., et al. (2017).
Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging.
Cell, 169(1), 132–147.e16.
https://doi.org/10.1016/j.cell.2017.02.031
Landmark study introducing FOXO4-DRI, demonstrating selective elimination of senescent cells and reversal of age-related and chemotherapy-induced tissue dysfunction in mice.
Supporting Senescence & Senolytic Research
Jeon, O. H., et al. (2017).
Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment.
Nature Medicine, 23, 775–781.
https://doi.org/10.1038/nm.4293
Demonstrates that removal of senescent cells improves tissue regeneration and reduces inflammation in vivo.
Farr, J. N., et al. (2017).
Targeting cellular senescence prevents age-related bone loss in mice.
Nature Medicine, 23, 1072–1079.
https://doi.org/10.1038/nm.4385
Shows that senescent cell clearance improves bone density and skeletal health, supporting senolysis as a therapeutic aging strategy.