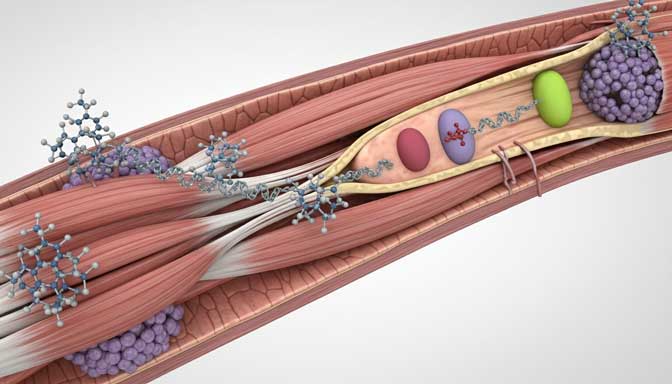
Follistatin-344 is a peptide increasingly recognized for its potential as a myostatin inhibitor, offering novel strategies for muscle growth and as an intervention against age-related muscle degeneration. As research into peptide muscle therapy expands, understanding the science behind Follistatin-344 and its applications for aging muscle becomes ever more critical. This comprehensive article evaluates current evidence, mechanisms, human studies, safety considerations, and practical implications for this compound in the context of muscle health and aging.
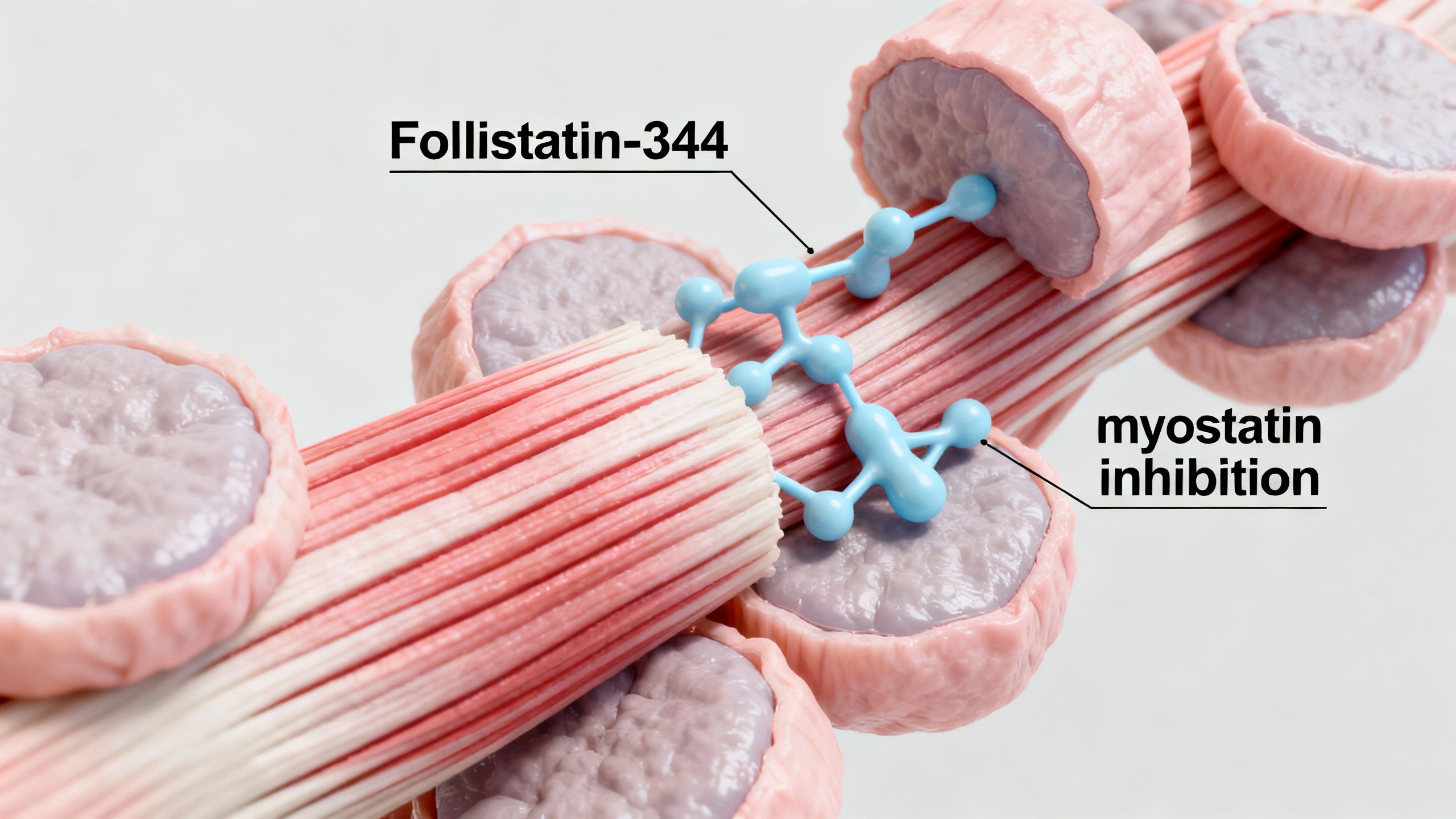
Understanding Follistatin-344: What Is It?
Follistatin-344 is a naturally occurring protein fragment designed to bind and neutralize myostatin—a regulatory protein that restricts muscle growth. By acting as a myostatin inhibitor, Follistatin-344 may open therapeutic avenues for muscle hypertrophy, particularly in individuals experiencing sarcopenia or other forms of muscle loss due to aging or chronic disease.
Biological Role & Relevance
- Follistatin family members regulate various biological processes, including muscle formation, via interactions with transforming growth factor-beta (TGF-β) proteins.
- Follistatin-344 is a specific isoform with a unique C-terminal region, enabling better tissue distribution and stability in circulation.
Mechanisms: How Follistatin-344 Inhibits Myostatin for Muscle Hypertrophy
Myostatin’s Role in Muscle Regulation
- Myostatin is a powerful negative regulator of muscle mass.
- High myostatin levels contribute to muscle atrophy, reduced regeneration, and impaired recovery—major factors in aging muscle decline.
- Inhibiting myostatin unleashes anabolic pathways, leading to enhanced protein synthesis and greater muscle fiber size.
Follistatin-344’s Mode of Action
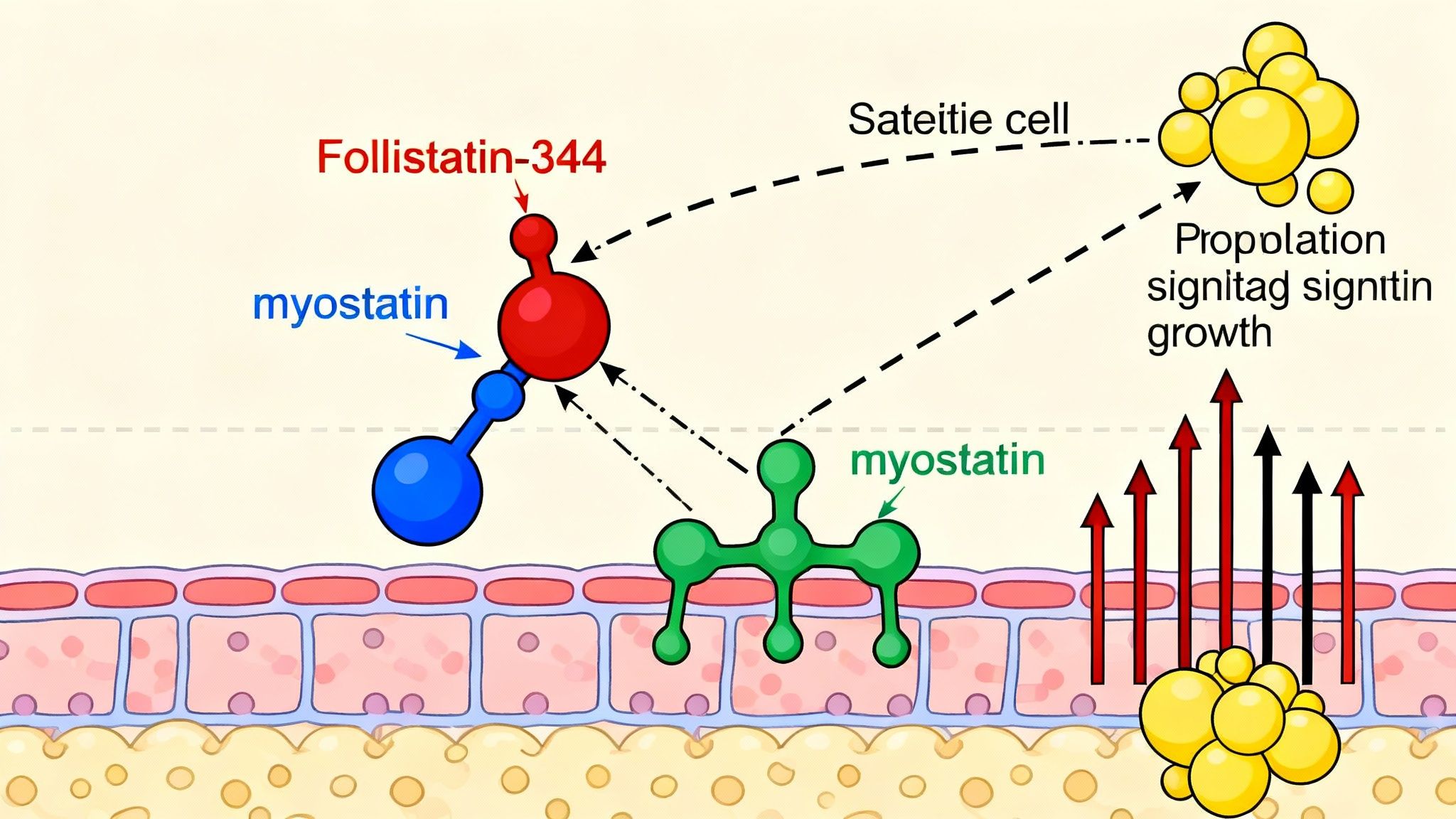
- Follistatin-344 binds directly to myostatin, preventing it from activating its receptor (ActRIIB) on muscle cells.
- This blockade disrupts the catabolic TGF-β signaling cascade, resulting in:
- Enhanced muscle hypertrophy
- Increased satellite cell activation and differentiation
- Potential reduction in inflammation-related muscle loss
Key molecular mechanisms include:
- Increased mTOR activity, essential for muscle protein synthesis
- Downregulation of transcription factors inhibiting muscle growth
Clinical Interest: Follistatin-344 in Human Muscle Health & Aging Intervention
Target Populations
- Sarcopenic elderly: Older adults experiencing age-associated muscle loss with frailty
- Patients with muscle-wasting diseases: Neuromuscular, metabolic, or cancer cachexia
- Athletes and bodybuilders: Exploring peptide therapy for muscle mass optimization (though such use is controversial and, in many contexts, unsanctioned)
Relevance to Sarcopenia and Aging Muscle
Sarcopenia, the degenerative loss of muscle mass with age, affects quality of life, mobility, and independence. Traditional interventions—such as resistance exercise and dietary protein—offer partial benefits but may not fully counteract severe muscle atrophy. Myostatin inhibitors like Follistatin-344 represent a promising experimental class of therapies to address this unmet need, either as a stand-alone intervention or as an adjunct to peptide therapy for muscle recovery.
Evidence from Human Studies: What Do We Know?
Current State of Research
While robust human clinical trials for Follistatin-344 are limited compared to animal and in-vitro studies, initial findings provide cautious optimism for its application in both aging intervention and muscle rehabilitation.
| Evidence Type | Status |
|---|---|
| Animal Models (mouse, pig, primate) | Strong muscle hypertrophy / mechanistic support (3) |
| Mechanistic Reviews | Clear myostatin/ActRIIB blockade rationale (5) |
| Human Clinical Data | Extremely limited or indirect — gene therapy approaches under exploration in research settings (not injectable peptide) |
Highlights from Human Research:
- Limited early-phase human trials have focused on safety, tolerability, and markers of muscle mass in select highly controlled populations.
- Some studies have evaluated systemic follistatin gene transfer rather than direct peptide administration, further complicating direct translation to peptide therapy.
What About Other Muscle Peptides?
For comparison, peptides like BPC-157, MOTS-c and TB-500 have shown therapeutic muscle repair effects in early research, suggesting a growing landscape of peptide muscle therapies.

Safety, Side Effects, and Medical Cautions
Potential Risks
- Unregulated muscle growth can, in theory, increase the risk of abnormal tissue development, including tumorigenesis, although data in humans remain very limited.
- Interference with normal TGF-β signaling could impact not only muscle, but also metabolic, cardiovascular, and reproductive health.
Known Side Effects (Preliminary Reports)
- Local discomfort at injection sites (for peptide administration)
- Possible impacts on glucose metabolism, fertility, or inflammation (based on related myostatin inhibitor studies)
- Unknown long-term risks—extreme medical caution and supervision are warranted
Regulatory Status
- Follistatin-344 is currently not FDA approved for any indication. Use is typically restricted to research settings.
Individual Variability and Limiting Factors
- Baseline muscle health, age, genetics, and concurrent therapies may all affect responsiveness to Follistatin-344.
- Some populations (e.g., those with active malignancies, or disorders of the TGF-β pathway) may face greater risks.
- Practical application is constrained by the need for controlled medical oversight and careful risk-benefit assessment.
Athletic enhancement with peptides is both ethically and medically controversial. For most people interested in muscle preservation and healthy aging, lifestyle interventions—such as resistance exercise and adequate protein intake—remain the mainstay, with potential adjunctive peptide therapies under investigation.

Practical Applications: Who Might Benefit?
- Older adults with sarcopenia seeking new approaches to prevent muscle loss
- Individuals recovering from severe injuries or illness associated with muscle atrophy
- Potential future use in clinical management of degenerative muscle disorders
- Note: Routine or unsupervised application is not recommended given the limited human evidence and unknown long-term outcomes
Comparison with Other Muscle-Related Peptide Therapies
- BPC-157: Promotes tissue healing; mostly studied in tendon and ligament repair
- TB-500: Similar regenerative properties, with broader tissue applications
- MOTS-c: Emerging mitochondrial peptide with reported benefits in metabolic health and endurance
Limitations, Unknowns, and Research Gaps
- Most available data comes from animal or gene therapy studies, not direct human peptide administration
- Optimal dosage, duration, and method of administration remain unclear
- Long-term safety and rare risks are not well-characterized
- Off-target effects on organs/systemic health are a major concern
Key caveat: Lack of long-term, large-scale clinical trials in typical aging adults or people with sarcopenia is a major limitation. Future research will need to address these gaps before widespread use.
Studies / References: Human Evidence Base Summarized
- Follistatin as a Myostatin Inhibitor: Gene therapy with FS344 increases muscle size and strength in animal models, demonstrating the translational potential of myostatin blockade.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2717722/ - Preclinical Mechanistic Evidence: Follistatin inhibits myostatin and activin signaling, increasing muscle weight and hypertrophy through satellite cell activation in rodents.
https://pubmed.ncbi.nlm.nih.gov/19435857/ - Muscle Mass Increase in Transgenic Animals: Overexpression of Follistatin-344 in pigs significantly increased skeletal muscle mass and reduced body fat.
https://pubmed.ncbi.nlm.nih.gov/27787698/ - Aged Mouse Model Muscle Improvement: Gene delivery of follistatin improved muscle weight and neuromuscular function in aged mice, relevant to sarcopenia research.
https://pubmed.ncbi.nlm.nih.gov/33964607/ - Myostatin Inhibition Strategy Review: Follistatin-based approaches show promise for inhibiting myostatin, though translational challenges and off-target considerations remain.
https://pubmed.ncbi.nlm.nih.gov/19208403/
Conclusion: The Future of Follistatin-344 in Muscle Health and Aging
Follistatin-344 holds substantial promise as a myostatin inhibitor for muscle hypertrophy, sarcopenia, and aging muscle intervention. However, its translation into human clinical practice is still in early days—marked by limited safety data, sparse randomized trials, and a need for greater scientific rigor. Anyone considering peptide muscle therapy should proceed within established clinical research protocols and in close consultation with qualified healthcare professionals.
As new evidence emerges, therapies like Follistatin-344 may offer new hope for healthy aging and muscle preservation. For now, staying informed and medically cautious is the best path forward.
Disclaimer: This article is for informational purposes only and not a substitute for professional medical advice. Consult a healthcare provider before making decisions about peptide or experimental therapies.